Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library
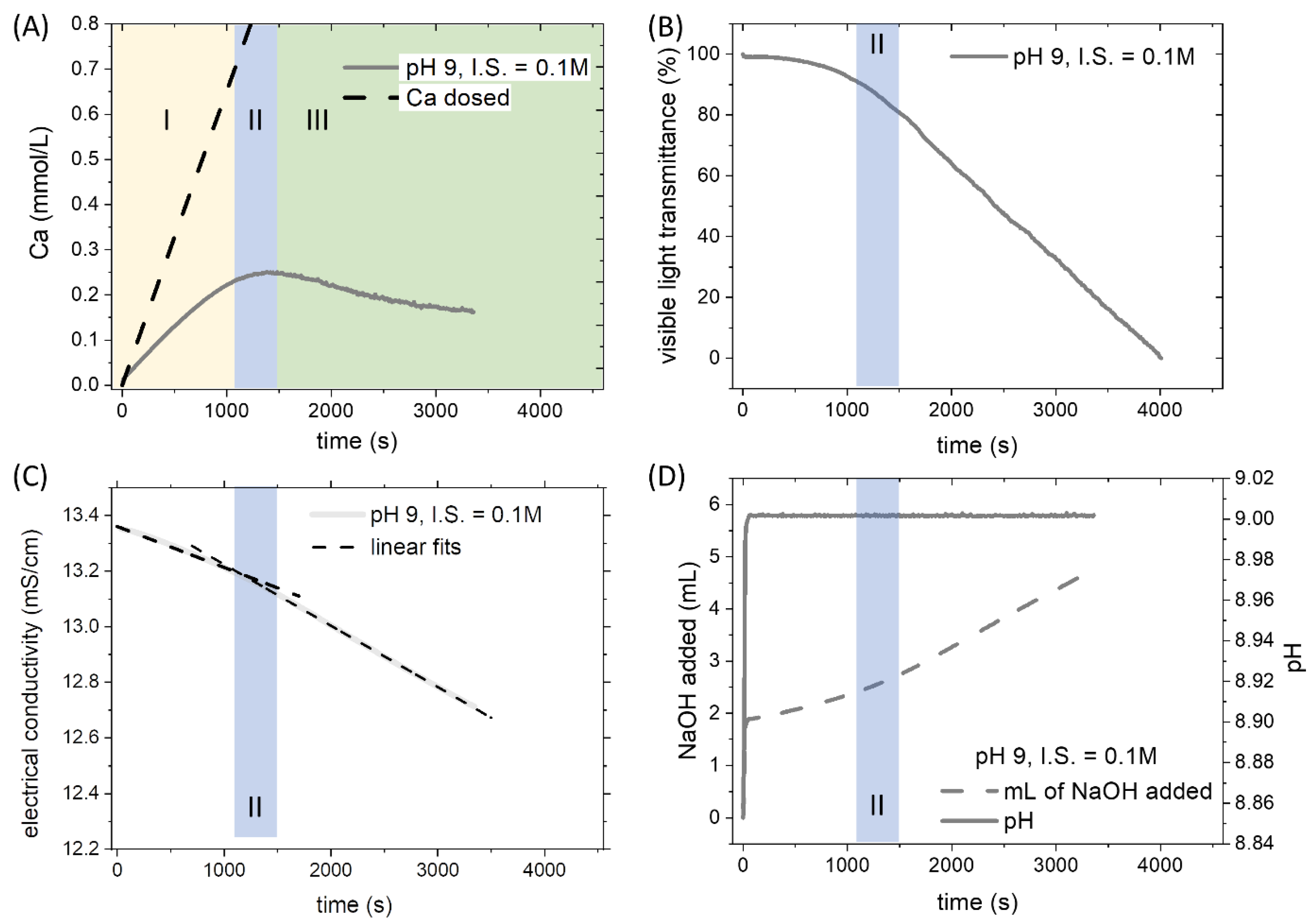
Minerals | Free Full-Text | The Effect of pH, Ionic Strength and the Presence of PbII on the Formation of Calcium Carbonate from Homogenous Alkaline Solutions at Room Temperature
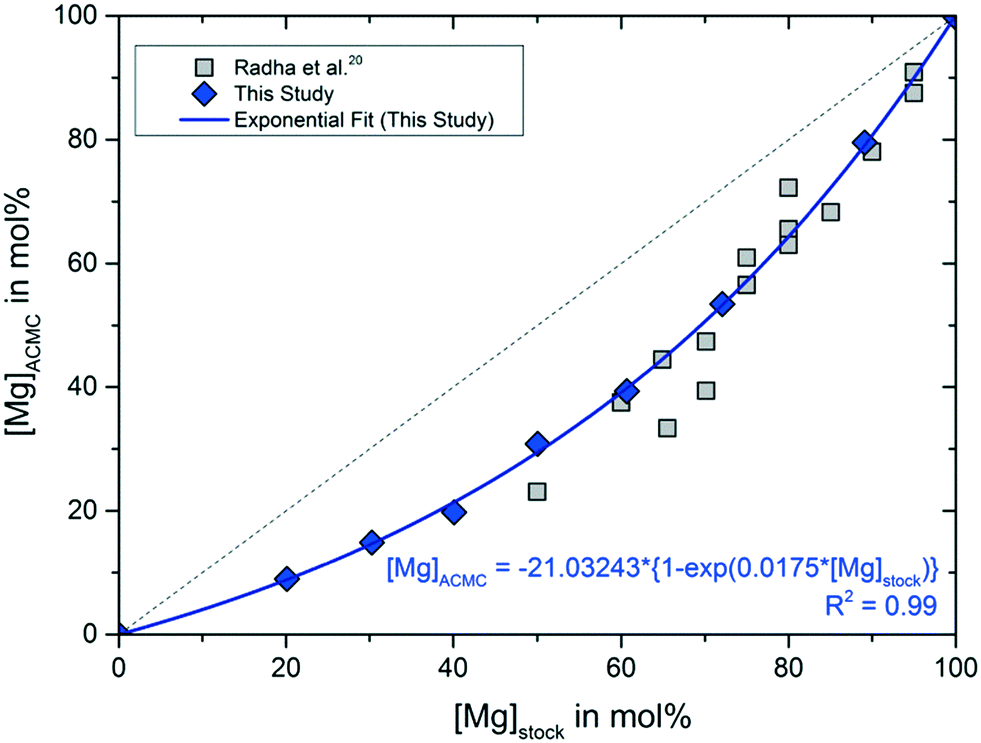
Solubility investigations in the amorphous calcium magnesium carbonate system - CrystEngComm (RSC Publishing) DOI:10.1039/C8CE01596A

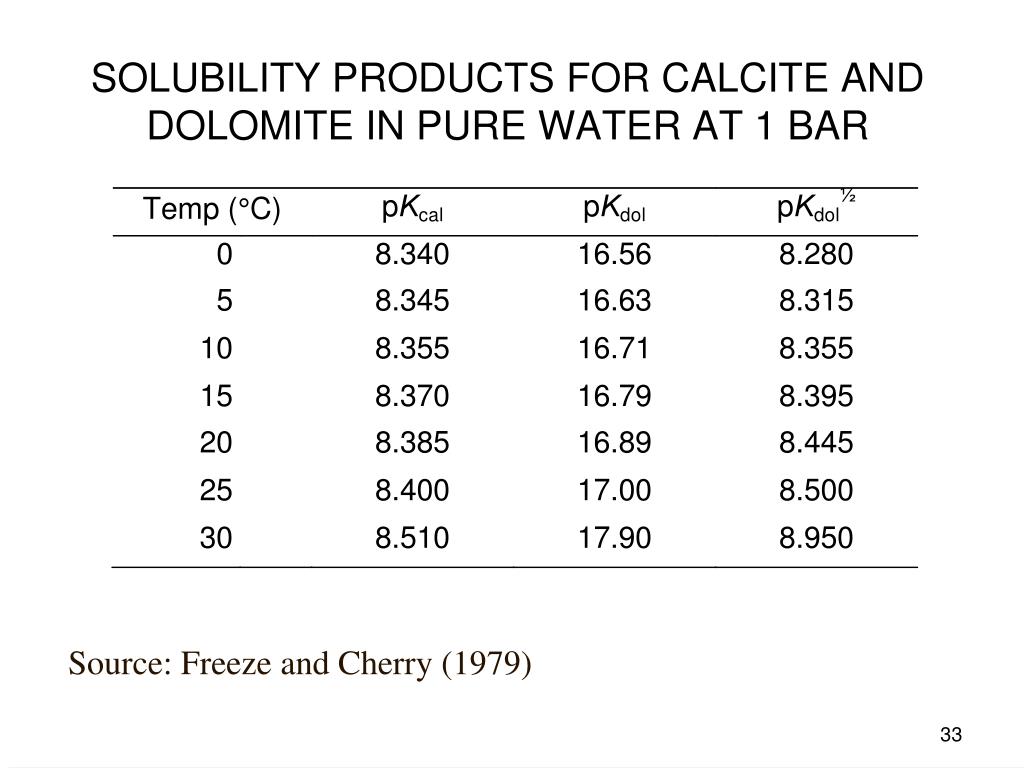
Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science
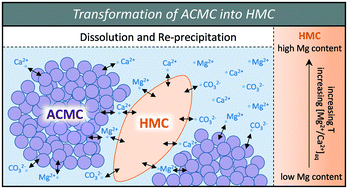
Effect of temperature on the transformation of amorphous calcium magnesium carbonate with near-dolomite stoichiometry into high Mg-calcite - CrystEngComm (RSC Publishing)

Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

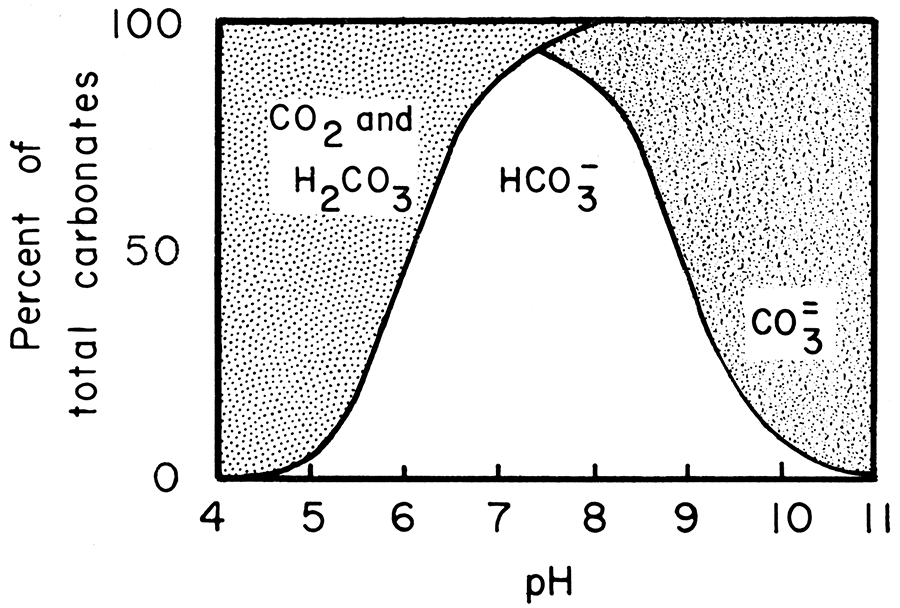
Saturation state of calcite and dolomite as a function of pH in the... | Download Scientific Diagram
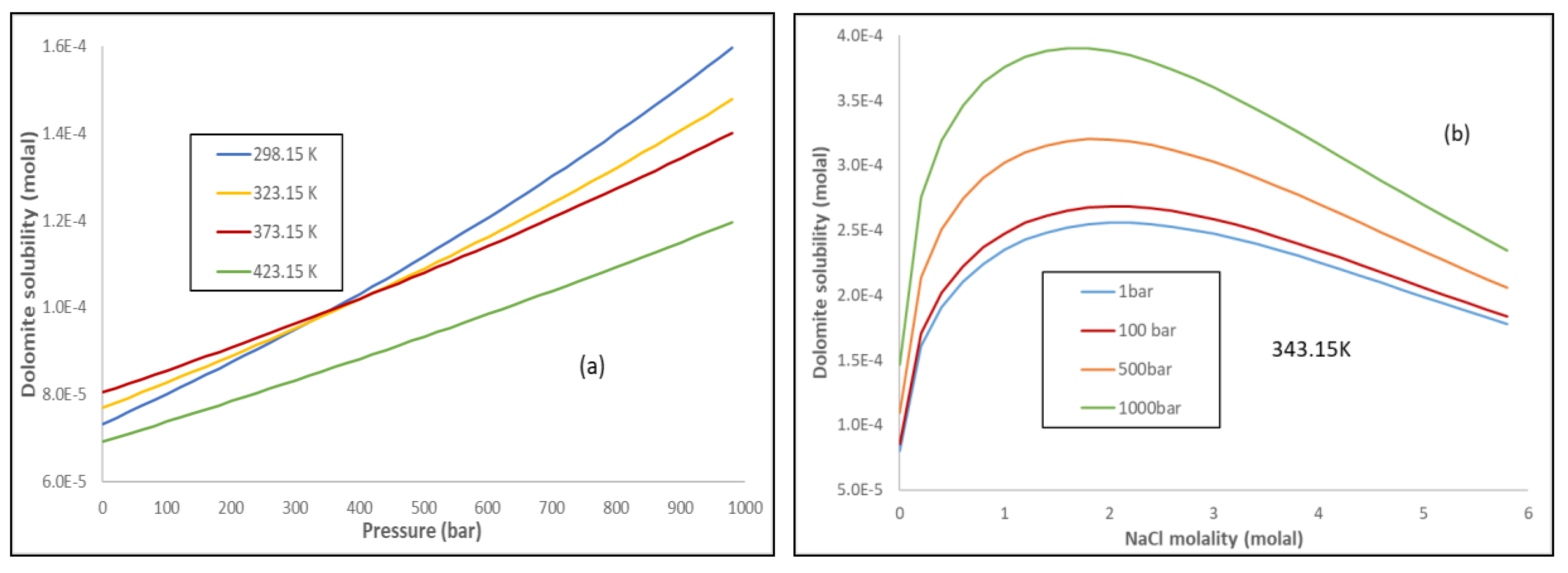
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure

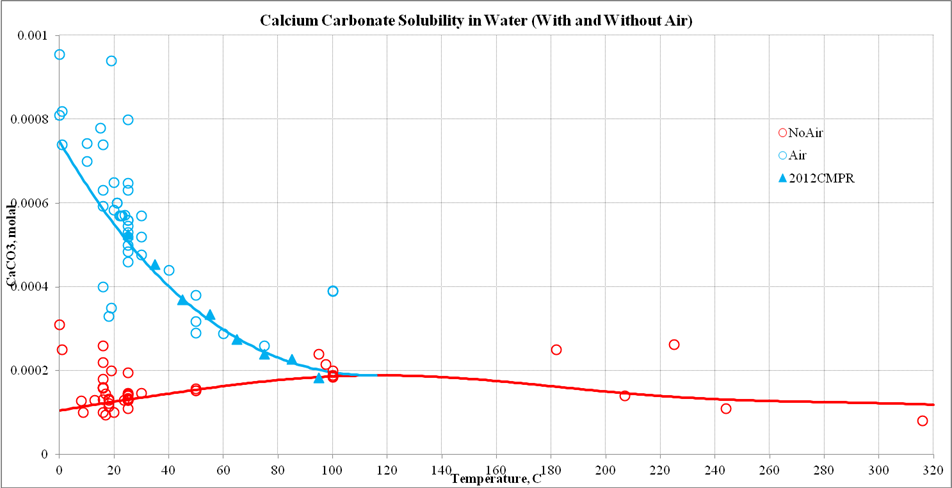
Calcite solubility in aqueous solution with CO2 in equilibrium obtained... | Download Scientific Diagram

An experimental study simulating the dissolution of gypsum rock - Dongdong Hong, Ming Fan, Lingjie Yu, Jian Cao, 2018
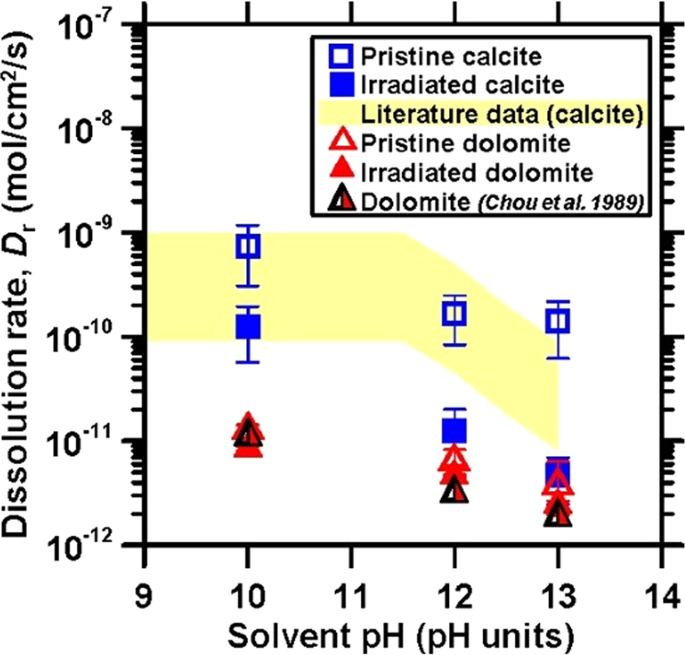
The effect of irradiation on the atomic structure and chemical durability of calcite and dolomite | npj Materials Degradation

5 Temperature Control of Mineral Deposition – A Conceptual Overview of Surface and Near Surface Brines and Evaporite Minerals

Self-accelerating volumetric dolomite-for-calcite replacement: A possible mechanism for high-temperature dolomitization? | SpringerLink

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega
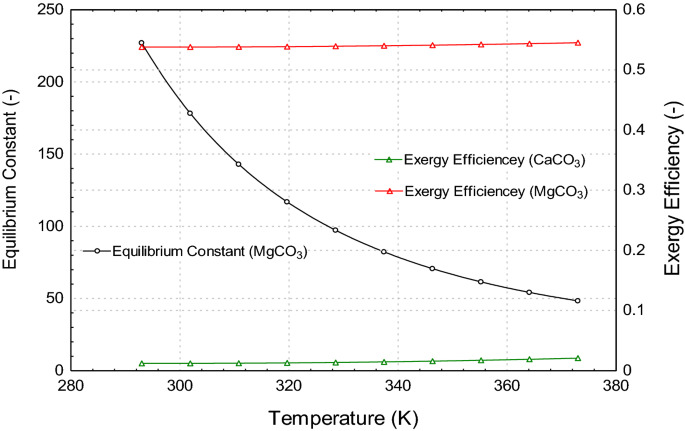
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega